Looking for Patient Care?
HIFU is a non-invasive, focal treatment for prostate cancer that allows precise application of heat to destroy cancer tissue. A probe is placed in the rectum for treatment, and high-energy ultrasound waves are focused on the prostate, which is 1-4 centimeters away. Importantly, the intervening tissue including the rectum is not heated. The treatment is radiation free and does not require incisions.
Aggressive or large prostate cancers are best treated with surgical removal or radiation, but smaller or less aggressive cancers are good candidates for treatment with HIFU. Some advantages of focal HIFU treatment include lower rates of urinary leakage (incontinence), better erectile function outcomes, and the ability to go home the same day. A urinary catheter may still be needed for a couple days, but this is less than the week typically required by surgical removal of the prostate.
Following FDA approval of Sonablate® in 2015, Indiana University School of Medicine Department of Urology faculty physicians became among the first to acquire the system to provide HIFU to patients as a focal treatment for prostate cancer, with the first device being built in Indianapolis in 1996. On April 26, 2000 a cancer symposium was held at Indiana University School of Medicine where the first research protocol was crafted for HIFU prostate treatment in the United States. Participants included Koch and Gardner as well as researchers from University Hospital Cleveland, Wake Forest University, University of Bern, University of Vienna and University of Amsterdam, among others.
In 2007, Koch with others at IU School of Medicine published on the first 20 patients treated with HIFU in United States. The study showed the treatment had few side effects and had the potential to be an effective treatment option for early-stage prostate cancer. They found that ablation of the whole gland can cause temporary swelling and the need for a temporary urinary catheter. This increased the interest in focal or partial-gland ablation. Subsequent studies on HIFU partial gland treatment have demonstrated preservation of erections in close to 90 percent and a 100 percent pad-free rate by three months.
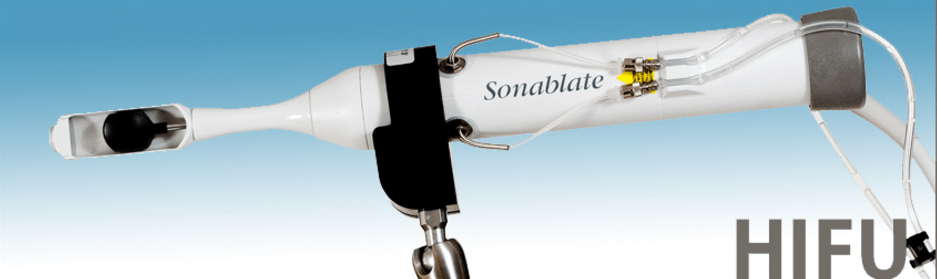
The first HIFU system (Sonablate®) was manufactured in Indianapolis in 1996 for a company called Focus Surgery. SonaCare Medical, LLC was established in 2013. Sonablate® became the first device approved by the FDA for HIFU in 2015. The video below includes more information about how the Sonablate® device works.
Video
Sonable HIFU Prostate Treatment 2016
Frequently Asked Questions
A probe is placed in the rectum for treatment, and high-energy ultrasound waves are focused on the prostate. The device contains an ultrasound for imaging to select regions for treatment and to monitor the effects of treatment in real-time. Sound waves are focused to heat tissue to nearly 200F, but the intervening tissue, including the rectum, is not heated. The process is analogous to using a magnifying glass to focus sunlight and heat an area.
A cooling system circulates chilled water throughout the procedure to prevent rectal heating and injury.
HIFU provides reliable cancer destruction in the targeted area without radiation or incisions, and the treatment has limited side effects, offering low risk of incontinence and erectile dysfunction. This treatment is an outpatient surgery procedure (2-4hours) with a short recovery.
Patients with early-stage prostate cancer that has not spread beyond the prostate are the best candidates for HIFU treatment. Prostate cancer that is localized to one side of the prostate on biopsy and MRI allows for focal treatment with fewer side effects. The treatment shows best results on small to medium-size prostate glands (height less than 4cm and volume <50cm3) and in patients who have excellent erectile and urinary function.
Cancer stage (clinical) is defined as:
- Discovered incidentally by PSA or by BPH surgery.
- Palpated on digital rectal exam and felt to be contained in the prostate.
- Palpated on digital rectal exam and felt to be extending through the prostate capsule.
- The tumor is invading local structures such as pelvic side wall.
Traditionally, the rectal exam, PSA blood test and prostate biopsy Gleason grade were used to predict the risk of cancer spreading beyond the prostate. However, some cancers extending beyond the prostate can be missed by these tests alone. Now, 3-Tesla multi-parametric MRI scans are used to better locate cancer and predict if it has spread outside the prostate.
HIFU is limited to treating cancer within the gland and cannot treat cancer spreading beyond the gland or into lymph nodes. In some cases, the surgical removal of the prostate and lymph nodes (e.g. robotic prostatectomy) can remove prostate cancer that has spread beyond the prostate.
The PSA test is checked periodically following treatment with HIFU or prostate removal. After HIFU, it should decrease significantly and remain stable with only slow rises over the rest of a patient’s life. A repeat biopsy is performed six months after HIFU to look for persistent cancer. After surgical removal of the prostate, the PSA should be undetectable. Prostate cancer that has spread outside the prostate (metastasized) is typically slow-moving and requires many years to cause symptoms and death. Due to this, if your life expectancy is less than 10 years, you might receive more harm than benefit from treatment.
Yes, in some cases an additional HIFU treatment can be given. Surgery and radiation can be used after HIFU as well.
HIFU was recently approved by the FDA and is not yet covered by insurance companies.
To learn more about HIFU or make an appointment with an IU School of Medicine faculty expert, visit the prostate cancer page on the IU Health website.

