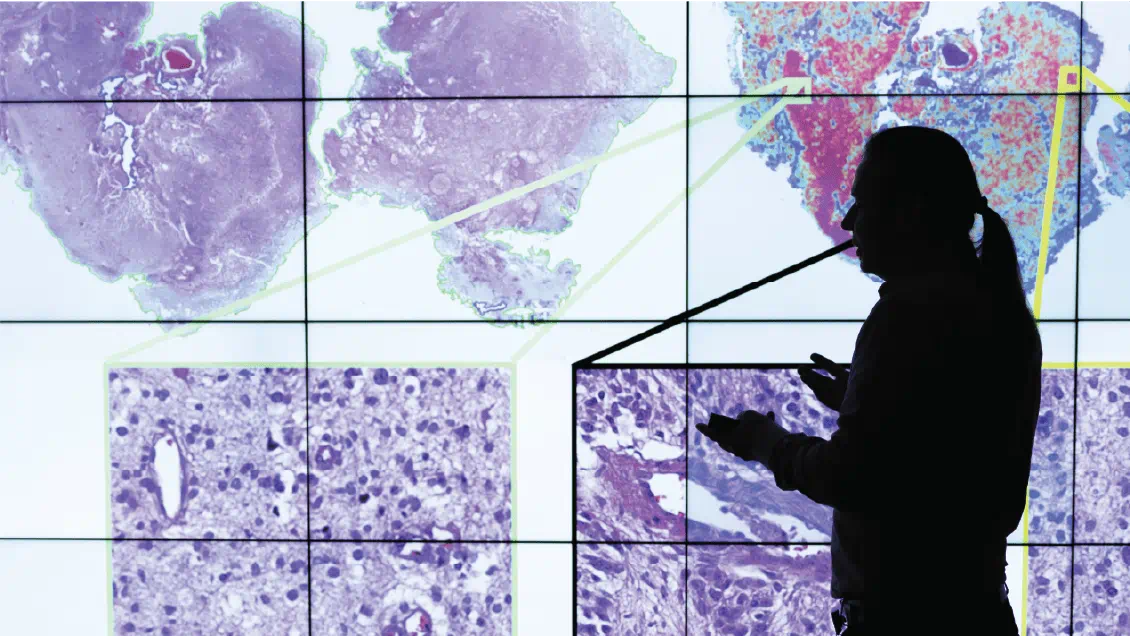
So, Alomari, an associate professor of clinical pathology and dermatology, set out with his lab to develop a tool to handle that task.
They started by using scanners to magnify curated pathology slides 400 times to create extremely high-resolution images that show all the cells in a sample. Those scanners simultaneously culled and fed data on pathological features into a supercomputer. “These are features where you can’t look at it in a matter of seconds and draw a conclusion,” Alomari said.
Analyzing the images means breaking them into thousands of small squares, and the algorithm evaluates the shape of cells in that quadrant, the coloration of certain features like the nucleus, and how many cells are packed into the space. Then, it slowly zooms out, quantifying spatial relationships from surrounding grids.
Next, the algorithm calculates a score based on those physical features and clinical outcomes. Statistically speaking, a higher score indicates a greater likelihood of a relationship. Once that training was done, Alomari’s team asked its algorithm to evaluate another set of images. It accurately gauged the outcome in 90% of cases.
Unlike some approaches, Alomari’s team can also outline how its machine-learning algorithm reached conclusions. “It’s also not a complete black box,” Alomari said. “You can express what feature the machine is looking at and why it’s associated with certain risks or certain biological behavior of melanoma.”
Some of the model’s conclusions weren’t all that surprising, either. The thickness of a melanoma tumor still matters. Yet the process also detected the kind of nuance that a human might miss. For example, the more diversity there is in the size of cells, the better the outcome tends to be for the patient.
“That might sound bad, but it means there are lots of immune cells mixed in with tumor cells,” Alomari said. “They haven’t been crowded out by cancer.”
In the near term, Alomari is focused on validating the model’s performance in Stage 1 and Stage 2 patients. Part of it is need. Those patients still make up most new cases. In patients with advanced skin cancer, teasing out relationships between physical features and clinical outcomes can be trickier. A patient’s treatment regimen, for example, may trip up the model.
But in time — and hopefully with backing from an NIH grant — Alomari thinks his team could find solutions. “That way we could predict your response and survival based on the type of additional treatment you got to lower the chances melanoma recurs.”
Donor support can scientists pursue bold research that improves care for melanoma. Learn more and make a gift today.