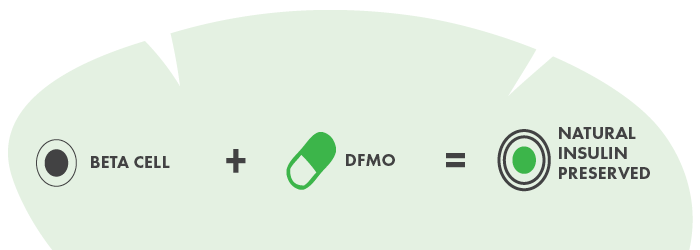
Diabetes researchers are studying a drug known as difluoromethylornithine (DFMO) that may reduce stress in cells that make insulin and preserve the body’s own insulin production.
The Targeting Type 1 Diabetes via Polyamines (TADPOL) trial is currently seeking participants who were diagnosed with Type 1 diabetes within the past 100 days. Researchers are trying to learn whether DFMO may be a useful treatment to improve beta cell health for persons with Type 1 diabetes.