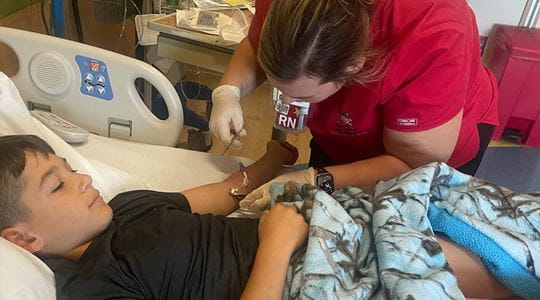
“Colin’s treatment is an exciting milestone of hope and perseverance,” said Jamie Felton, MD, assistant professor of pediatrics and physician scientist with the Center for Diabetes and Metabolic Diseases (CDMD) and the Herman B Wells Center for Pediatric Research at IU School of Medicine. “The first study using this drug in type 1 diabetes in animal models was published in 1993, so it took decades of research and resources to get to this point.”
Teplizumab is an antibody that disrupts the communication between the immune cells that mistakenly destroy the insulin-producing beta cells that affect blood sugar levels in diabetes patients. It is administered through a two-week daily infusion.
Colin’s parents knew he was a good candidate for the therapy because they had him screened due to their family history with the disease—two of his older sisters have type 1 diabetes. While the treatment is not intended to permanently prevent a diagnosis, the Ozdemirs and diabetes experts agree delaying the disease by at least a couple of years is an incredible advancement that improves the quality of life for those at risk.
Dr. Felton oversaw Colin’s treatment as director of a new early-stage diabetes clinic at Riley Hospital for Children where she will continue to monitor his progress and treat other at-risk or newly diagnosed pediatric patients. She hopes more families like the Ozdemirs will come forward for screenings or volunteer in similar clinical trials and anticipates the new diabetes clinic will continue to grow as screening for type 1 diabetes is expanded.
 "The goal of the early stage type 1 diabetes clinic is to provide a medical home for people with risk for type 1 diabetes, but do not yet need insulin," said Dr. Felton. "In the clinic, the patients will be monitored for disease progression and be made aware of both research and treatment opportunities, such as teplizumab, as they become available."
"The goal of the early stage type 1 diabetes clinic is to provide a medical home for people with risk for type 1 diabetes, but do not yet need insulin," said Dr. Felton. "In the clinic, the patients will be monitored for disease progression and be made aware of both research and treatment opportunities, such as teplizumab, as they become available."
The IU School of Medicine and Riley Hospital pediatric endocrinology team has several clinical studies underway focused on type 1 diabetes prevention and beta cell preservation. The clinical research team led by Linda DiMeglio, MPH, MD, is part of Type 1 Diabetes TrialNet, an international network of specialists at the forefront of type 1 diabetes research that also organized the initial teplizumab study in 2019.
“Even though teplizumab is FDA-approved and available to the public, we’re still carefully analyzing its effects and investigating its potential,” said Emily K. Sims, MD, associate professor of pediatrics and key investigator in the teplizumab studies. “Our latest study suggests there may be strategies to identify the optimal treatment time in patients at risk before it’s too late.”
Sims and her colleagues recently published their latest teplizumab findings in Diabetologia and will continue to study the drug and other potential therapies for type 1 diabetes.
As IU School of Medicine researchers diligently analyze the effects of teplizumab and explore new strategies for disease prevention, their work offers hope to countless individuals and families affected by diabetes. Through their investigations, clinical trials and the establishment of specialized clinics like the early stage type 1 diabetes clinic, they are making significant strides in improving the quality of life for those at risk.
To learn more about type 1 diabetes clinical trial opportunities, visit the type 1 diabetes clinical research website.