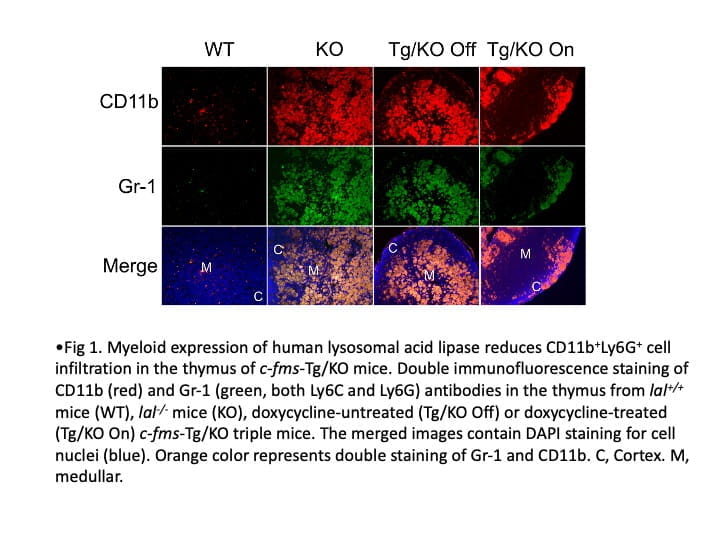
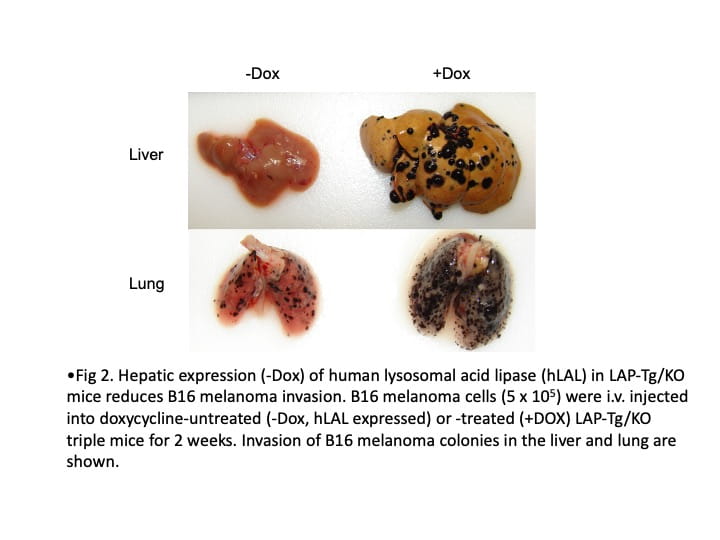
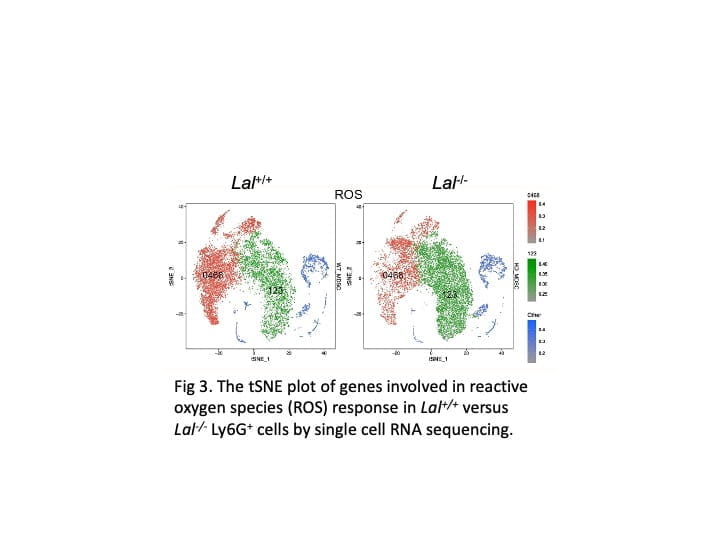
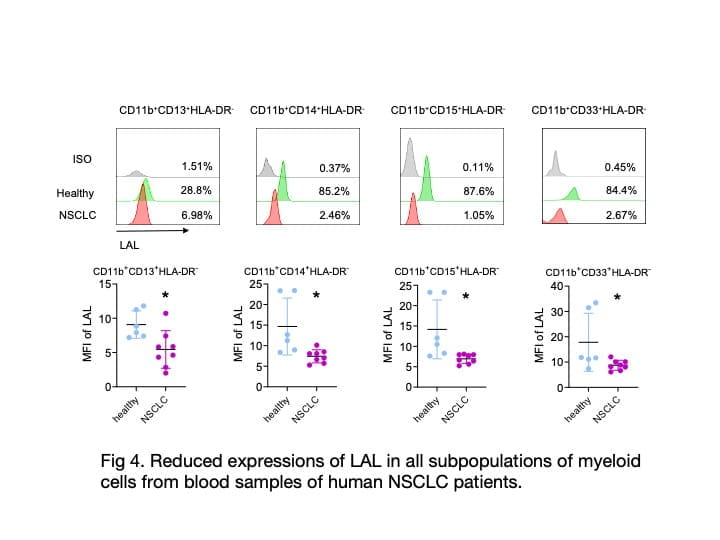
The primary research focus in my laboratory is to identify the cellular and molecular mechanisms by which inflammatory cells stimulate tumor cell proliferation, progression, and metastasis. We actively seek new immunotherapeutic avenues to treat cancers. My laboratory has been studying lysosomal acid lipase (LAL) for thirty years. We were the first group to clone the mouse LAL gene and created the knockout mice (lal-/-). Since then, LAL has continued to yield fascinating and surprising results in numerous research areas. Deficiency of LAL resulted in increased expression of CD11b+Ly6G+ myeloid-derived suppressor cells (MDSCs), and expression of human LAL in myeloid cells can reduced CD11b+Ly6G+ cells in Thymus of c-fms-Tg/KO mice (Fig 1). CD11b+Ly6G+ cells from lal-/- mice can stimulate B16 melanoma cells proliferation in vitro, tumor growth in vivo, enhance tumor cell invasion, and suppress T cell proliferation and function. Expression of human LAL in hepatocytes reduces B16 melanoma cell invasion in the liver and lung of LAP-Tg/KO mice (Fig 2). By single cell RNA sequencing of CD11b+Ly6G+ cells from lal+/+ and lal-/- mice, data showed the increased expression of genes response to reactive oxygen species (ROS) in cell cluster 123 of lal-/- mice (Fig. 3). Furthermore, we detected a significant decreased expression of LAL in all subpopulations of myeloid cells from blood samples of human non-small cell lung cancer (NSCLC) patients compared to healthy individuals (Fig 4). These findings provide a strong foundation that LAL can serve as a modulator for immune cells’ anti-tumor function.
Most recently, we demonstrated that the LAL-controlled neutral lipid metabolic signaling pathway plays a critical role in regulating the development and homeostasis of CD11c+ myeloid cells. We have shown that CD11c+ myeloid cells with LAL deficiency not only possess strongly immunosuppressive function but also directly stimulate the proliferation of several cancer forms (melanoma, lung cancer, liver cancer, breast cancer, prostate cancer, etc.). During these studies, we identified that CSF1R, PD-L1 and metabolic reprogramming mediate LAL functions in tumor immunology. Our current research aims to identify new molecules and pathways controlling the anti-tumor and protumor functions of CD11c+ myeloid cells in the tumor environment. The outcome of our research is to enhance anti-tumor immunity by altering the immune cell function through metabolic enzyme like LAL and other downstream regulators such as CSF1R and PD-L1.
Dr. Du is a co-inventor for multiple patents that granted by US Patent and International Patent Offices:
"Use of Lysosomal Acid Lipase for Treating Atherosclerosis and Related Diseases"
PCT/US2001/003481.
Inventors: Gregory A. Grabowski and Hong Du
International Patent Office, 09/09/2002
“Lipid Hydrolysis Therapy for Atherosclerosis and Related Disease”
US patent No.: US 6,849,257 B2
Inventors: Gregory A. Grabowski and Hong Du
US Patent Office, 02/01/2005
“Gene-based Lipid Hydrolysis Therapy for Atherosclerosis and Related Disease”
US Patent No.: US 11,653,147
Inventors: Gregory A. Grabowski and Hong Du
US Patent Office, 11/05/2007
“Methods for Treating Lysosomal Acid Lipase Deficiency”
US Patent No.: US 61,443,079
Inventors: Gregory A Grabowski, Hong Du, Michael Heartlein, Michael Concino, Paolo Martini, Muthuraman Meiyappan, Alla Romashko, Brian Pescatore, Lawrence Charnas.
US Patent Office, 08/15/2013
“Transgenic Mouse Conditionally Expressing MMP12 for Studying Myelopoiesis, Immunity and Tumorigenesis”
US Patent No.: US 9,279,117
Inventors: Cong Yan and Hong Du
US Patent Office, 03/08/2016
“Compositions and Methods for Diagnosing Lung cancer”
US Patent No.: US 10,782,297. B2
Inventors: Cong Yan and Hong Du
US Patent Office, 09/22/2020
“Lysosomal Acid Lipase and PPARGamma ligands as an Immune Therapies for Cancer Treatment”
US Patent No.: US 10,857,209
Inventors: Cong Yan and Hong Du
US Patent Office, 12/08/2020
“Lipid Hydrolysis Therapy for Atherosclerosis and Related Disease”.
US Patent No.: US 10,864,255
Inventors: Gregory A. Grabowski and Hong Du
US Patent Office, 12/15/2020
“Targets and Biomarkers for Tumor Microenvironments”
US Patent Application No.: 18/599,502
Inventors: Cong Yan and Hong Du
Filed: 03/08/2024