Meichen Yu, PhD, specializes in multimodal neuroimaging (MRI, EEG/MEG) and brain network (connectome) analyses in dementia and mood disorders. His research focuses on the genetic architecture of brain structural and functional networks in Alzheimer’s disease (AD) and multilayer structure-function connectome changes in AD. Specifically, he investigates associations between the human connectome (measured by functional and diffusion MRI), amyloid-β and tau pathologies (measured by PET), blood-based transcriptomic profiles, genomic variations, neurotransmitter receptor density maps (derived from PET), and cognitive decline, as well as neuropsychiatric syndromes in AD.
Identifying the Role of the Transcriptome in Connectome Disruption in AD
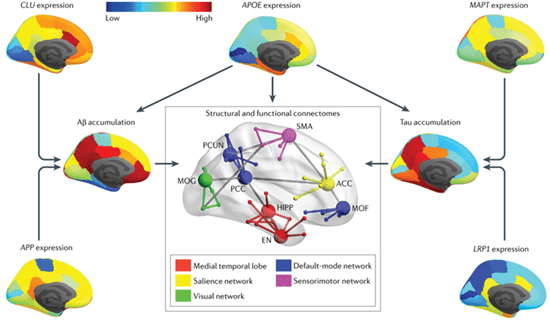
The Yu Lab investigates the genetic architecture and neuropathological mechanisms underlying human connectome disruption in AD. A recent study from his group examined how the connectome evolves across different stages and phenotypes of AD by synthesizing empirical data on connectome alterations and linking them with established biomarkers, including amyloid-β (Aβ), tau, and neurodegeneration, as well as genetic variation. This work also underscored the value of whole-brain computational models for tracking and predicting Aβ deposition and tau propagation.
In another study, the lab identified spatial associations between brain-wide gene expression profiles and regional patterns of Aβ and tau pathology. Additionally, using tau PET data, a novel AD risk locus was recently discovered. In ongoing work, the lab has also linked aberrantly expressed non-coding RNAs (ncRNAs) and altered post-translational modification (PTM) events with AD traits, and is leveraging these findings to construct regulatory networks involving relevant protein-coding genes.
Discovering Reproducible Multi-Dimensional Neuroimaging Mechanisms for Neuropsychiatric Symptoms in AD
Dr. Yu’s work has advanced understanding of neuropsychiatric symptoms (NPS) in AD through integrative analyses of multidimensional neuroimaging and molecular data. By combining biomarkers of amyloid-β and tau pathology (PET), structural and functional connectome disruptions (MRI), regional gene expression (transcriptomics), and neurotransmitter receptor density maps, his research supports the hypothesis that these A/T/N/G/R factors jointly contribute to NPS and cognitive decline in preclinical and prodromal AD. This work draws on data from large-scale cohorts including IMAS, ADNI, and KBASE, as well as open-access resources such as the Allen Human Brain Atlas and the Neurotransmitter Database.
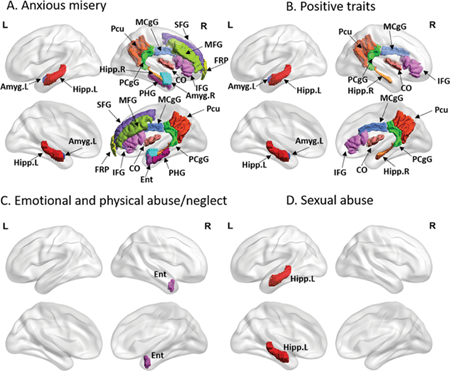
Additionally, Dr. Yu has explored the role of anxious-depressive symptoms (ADS) and sleep disturbances (SD) as both manifestations and potential risk factors for AD progression. His work integrates neuroimaging with behavioral and genetic data to propose dynamic models linking ADS, SD, and AD biomarkers, and highlights the potential of precision medicine approaches to address clinical heterogeneity in AD.
Earlier studies by Dr. Yu and collaborators identified robust associations between childhood trauma, dimensional symptoms, and altered network architecture in major depression using functional MRI. Follow-up structural MRI studies used multivariate methods to map brain-behavior relationships across clinical domains, including anxiety, depression, personality traits, and trauma history, and replicated these findings using cross-validation techniques. Together, these studies provide compelling evidence for the role of connectome disruption in neuropsychiatric syndromes and their overlap with AD risk.
Identifying MEG-based Brain Network Biomarkers in Aging and AD
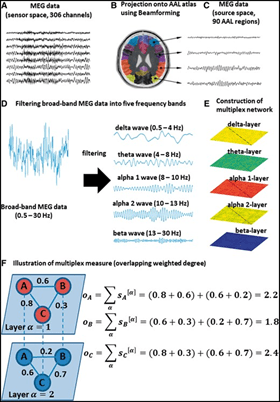
Dr. Yu has contributed significantly to the development and application of novel connectivity metrics in MEG-based studies of aging and AD. In a landmark 2016 study—the first MEG-based multilayer connectome analysis in AD—his team identified selectively vulnerable hub regions in the hippocampus, posterior default mode network, and occipital cortex. This work revealed that hub vulnerability was associated with both impaired cognition and abnormal cerebrospinal fluid amyloid-β42 levels. Importantly, the multilayer centrality metrics used in this study successfully captured posterior midline and hippocampal hubs typically missed in frequency-specific MEG analyses. The findings, published and highlighted by Brain, established a framework for integrating frequency-dependent networks and illuminated mechanisms of hub disruption in AD.
Subsequent work explored frequency-specific information flow in MEG-derived brain networks. In healthy adults, Dr. Yu’s team identified a posterior-to-anterior pattern of information flow in the alpha and beta bands, driven by visual cortex and posterior DMN hubs, alongside an anterior-to-posterior theta-band pattern originating from frontal regions. In contrast, MEG data from AD patients showed disruption of the beta-band posterior-to-anterior flow, suggesting that AD pathology alters directional information transfer, particularly from posterior hubs.
To address limitations in conventional directed connectivity metrics, which often assume periodic input signals, Dr. Yu developed a novel, robust measure for estimating the direction of information flow between time series. This method accommodates non-periodic signals and is resilient to variability in coupling strength, time delays, sample size, noise, spatial leakage, and EEG reference schemes, making it a promising tool for analyzing complex electrophysiological and neuroimaging data.
Current Research Funding
Identifying the Role of Transcriptome in Connectome Disruption of AD
Active
AARF-22-722571
Role: PI
09/01/2022 – 08/31/2025
Discovering Reproducible Multi-Dimensional Neuroimaging Mechanisms for Neuropsychiatric Symptoms in Alzheimer's Disease
Pending
NIH
Role: PI
04/01/2025 – 03/31/2030
Building A Novel Multilayer Structural-Functional Connectome Model to Advance Understanding of Pathology Propagation in Alzheimer's Disease
Pending
NIH
Role: PI
04/01/2025 – 03/31/2030
Identifying Reproducible Brain Connectome Changes in Alzheimer's Disease
Pending
NIH
Role: PI
12/01/2025 – 11/30/2030
Recent Publications
Yu M, Sporns O, Saykin AJ*. The human connectome in Alzheimer disease –– relationship to biomarkers and genetics. Nat Rev Neurol 2021; 17:545–563. PMID: 34285392; PMCID: PMC8403643.
Chai Y, Sheline YI, Oathes DJ, Balderston NL, Rao H, Yu M*. Functional connectomics in depression: insights into therapies. Trends Cogn Sci 2023; 27(9):814-832. PMID: 37286432; PMCID: PMC10476530.
Yu M*, Risacher SL, Nho KT, Wen Q, Oblak AL, Unverzagt FW, Apostolova LG, Farlow MR, Brosch JR, Clark DG, Wang S, Deardorff R, Wu Y, Gao S, Sporns O, Saykin AJ*, for the ADNI; Spatial transcriptomic patterns underlying amyloid-β and tau pathology are associated with cognitive dysfunction in Alzheimer’s disease. Cell Rep 2024; 43(2):113691. PMID: 38244198.
Nho K, Risacher SL, …, Yu M, …, Saykin AJ. CYP1B1-RMDN2 Alzheimer’s disease endophenotype locus identified for cerebral tau PET. Nat Commun 2024; 15(1):8251. PMID: 39304655; PMCID: PMC11415491.
“Chai Y, Shokri-Kojori E, Saykin AJ, Yu M*. Anxious–depressive symptoms and sleep disturbances across the Alzheimer disease spectrum. Nature Mental Health 2025. https://www.nature.com/articles/s44220-025-00416-4
Research Team
Luka Cvijanovic
Meiheng Liang
Hongyu Zou
Joshua Cochran
