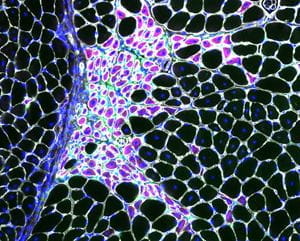
Macrophages (green) closely associated with regenerating fibers (purple) in dystrophin-deficient skeletal muscle.

Macrophages (green) closely associated with regenerating fibers (purple) in dystrophin-deficient skeletal muscle.
Duchenne muscular dystrophy (DMD) is a devastating muscle disease caused by genetic mutations to the dystrophin gene. Without dystrophin the mechanical infrastructure of striated muscle cells is compromised causing destabilization of the muscle cell membrane and cellular necrosis. While membrane fragility contributes to the pathophysiology of DMD, it alone cannot account for many pathological features of the disease. The pathophysiology of dystrophin-deficiency is intertwined with multiple secondary defects, including perturbations in the epigenetic regulation of gene expression, as well as considerable involvement of other tissues such as the immune system and stromal cells. Patients could benefit from effective countermeasures that target events downstream of dystrophin-deficiency. Our findings have shown that ⍺Klotho is epigenetically silenced in dystrophic muscles at the onset of dystrophinopathy and that restoring ⍺Klotho expression systemically or via infiltrating bone marrow-derived cells improves regeneration and function. Ongoing investigations, supported by the Muscular Dystrophy Association, are aimed at investigating the mechanisms through which restoring ⍺Klotho reduces fibrosis in dystrophic muscles.
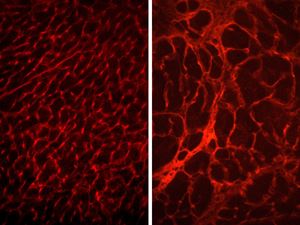
Pathological remodeling of the dystrophin-deficient myocardium (right).
Inflammatory lesion in injured muscle featured on the cover of The Journal of Immunology (Vol. 205, Issue 6. DOI: 10.4049/jimmunol.2000247)
