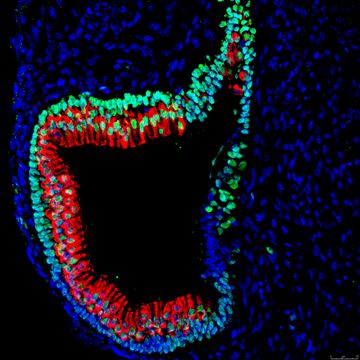
The Nelson Lab, led by Rick Nelson, MD, PhD, is interested in elucidating the mechanisms that underlie hair cell degeneration as it relates to deafness and balance disorders.
 Active Research
Active Research
This laboratory team currently focuses on why serine proteases are critical for the survival of hair cells in the inner ear and what cellular pathways mediate hair cell degeneration and deafness. Currently, the goal of this research is to understand how type II transmembrane serine protease 3 (TMPRSS3) functions, to identify and define the mechanisms that lead to hair cell degeneration and to develop therapeutic strategies for deafness and balance disorders.
Loss of mechanosensitive hair cells in the inner ear is a leading cause of profound hearing loss and balance disorders, affecting millions of people. Human inner ear hair cell fail to regenerate once they are lost. To date, more than 110 genes have been implicated in causing autosomal recessive congenital deafness and most of these gene mutations lead to degeneration of inner ear hair cells. The Nelson Lab aims to increase knowledge on how hair cell degenerate due to genetic mutations. Elucidating the mechanisms of hair cell degeneration is crucial to the treatment of inner ear disorders.
Human mutations in TMPRSS3 leads to autosomal recessive deafness (DFNB8/10). Mouse mutants of Tmprss3 (Tmprss3Y260X) show normal development of hair cells followed by rapid degeneration of hair cells over 48 hours. The mechanism of why TMPRSS3 is critical for hair cell survival is currently unknown.
In the Nelson Lab, we use a novel stem cell derived 3D inner ear organoids and mutant mice to study the mechanisms of genetically-mediated hair cell degeneration. The lab also uses CRISPR/Cas9 gene editing, single cell RNA-seq and proteomics to determine the cellular pathways impacted by deafness causing gene mutations.