Dr. Liu’s lab is dedicated to employing systems biology approaches to decipher the molecular mechanisms of gene regulation that play critical roles in complex diseases such as cancer, addiction, and neurodegenerative disorders. Specific research areas include developing and implementing cutting-edge experimental assays and innovative computational algorithms in understanding the functions of genetic variants in complex diseases, designing methodologies on analyzing multimodal high-dimensional data, and understanding regulatory mechanisms and translational impact of alternative splicing in cancer and other diseases. All these areas involve multi-disciplinary components, including functional genomics, genetics, computational and statistical modeling, computer science/engineering, and data management.
Research
Developing innovative experimental and computational methods for causal gene identification in complex diseases and for supporting precision medicine
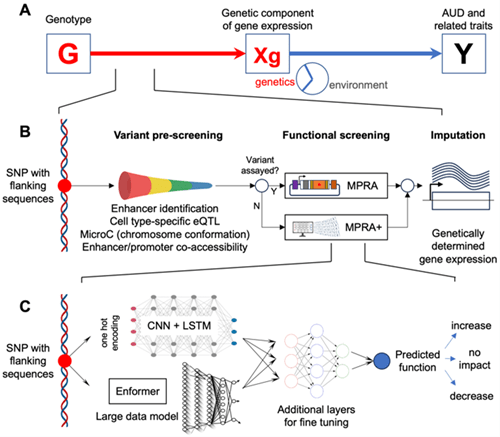
Recent advances in genetics and functional genomics provide exciting opportunities to identify the influence of genetic variation in complex disorders. However, the still limited power of genome-wide association studies (GWAS) for these disorders, combined with the difficulty of identifying functional variants in associated loci, has limited progress. Even when well powered, GWAS identify loci that span many variants, even after follow-up fine mapping studies. This has greatly hampered the identification of functional variants that contribute to the risk for complex diseases, and thereby the understanding of pathways and mechanisms involved in their etiology. A partial solution to this problem is the use of massively parallel reporter assays (MPRA) to systematically profile the activity of large numbers of regulatory variants in cell types that are relevant to a specific disorder. The functional information generated can be used to increase the ability of GWAS to identify relevant variants and to point toward their mechanism of action. We have developed MPRA that simultaneously test the functional activity of over 10,000 variants in the 3’-untranslated regions (3’UTR) and enhancer regions (Frontiers in Genetics, 2018, Alcohol Clin Exp Res., 2020, Molecular Psychiatry, 2021, bioRXiv, 2024). We have also developed a series of AI and machine learning models for identifying causal genes contributing to a specific disorder by combining MPRA-derived functional data with GWAS data.
The strategy has been applied to substance use disorder in a funded R01 grant with NIDA and a U10 grant funded by the NIAAA. The method and strategy we have developed can be readily utilized in other disease systems. We are currently developing grant proposals for identifying causal genes in Alzheimer’s disease, and to identify functional somatic mutations in cancer.
Roles of alternative splicing in complex disease
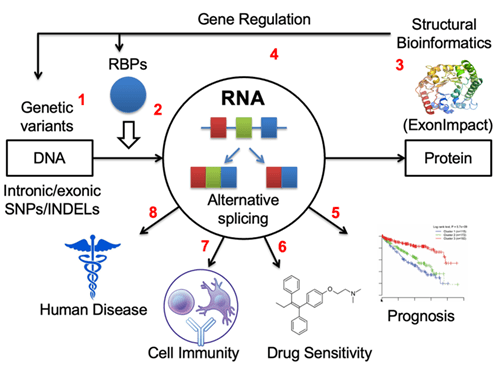 Over the past 15 years, I have made significant progresses in understanding the splicing regulation and its translational impacts in several aspects, including identify cis-acting RNA elements from CLIP-seq assay by innovatively integrating RNA sequence with secondary structure features (RNAMotifModeler, BMC Genomics 2011), extract novel alternative splicing events from RNA-seq data (Alt Event Finder, BMC Genomics 2012), predicting the pathogenic impacts of the dysregulation of splicing outcomes (ExonImpact, Human Mutation, 2017), discover the roles of exonic and intronic variants on splicing regulation (regSNPs-splicing, Human Genetics, 2017, and regSNPS-intron, Genome Biology, 2019), and examining the correlation between aberrant splicing patterns and cancer patient survival outcomes (Frontiers in Genetics, 2020) as well as the relationships between splicing regulation and drug resistance (Genomics, Proteomics and Bioinformatics, 2022).
Over the past 15 years, I have made significant progresses in understanding the splicing regulation and its translational impacts in several aspects, including identify cis-acting RNA elements from CLIP-seq assay by innovatively integrating RNA sequence with secondary structure features (RNAMotifModeler, BMC Genomics 2011), extract novel alternative splicing events from RNA-seq data (Alt Event Finder, BMC Genomics 2012), predicting the pathogenic impacts of the dysregulation of splicing outcomes (ExonImpact, Human Mutation, 2017), discover the roles of exonic and intronic variants on splicing regulation (regSNPs-splicing, Human Genetics, 2017, and regSNPS-intron, Genome Biology, 2019), and examining the correlation between aberrant splicing patterns and cancer patient survival outcomes (Frontiers in Genetics, 2020) as well as the relationships between splicing regulation and drug resistance (Genomics, Proteomics and Bioinformatics, 2022).
Recently, we and others have found that a type of dysregulated RNA splicing events, intron retention (IR), have been commonly observed in tumor transcriptomes. IR occurs when the splicing complex fails to splice introns from the primary messenger RNA transcript and trigger immune responses by either producing disease-specific neopeptides (neoantigens) that can be presented by MHC class I molecules, or generating pseudo-viral response by forming double-stranded RNA molecules. We have published two papers recently concluding that the levels of aberrant intron retention are associated with prognoses in multiple myeloma (Oncogene, 2021) and pancreatic cancer (JCO Clinical Cancer Informatics, 2022). Our preliminary data based on samples collected at IUSCCC precision clinic suggests that patients with higher intron retention levels tend to respond better to the immune checkpoint inhibitor therapy.
In addition to cancer, we recently discovered that aberrant intron retention is also present in other complex diseases such as Alzheimer’s disease, alcohol addiction, alcoholic liver disease, and diabetes. We have also designed a statistical genetics model to identify splicing patterns that contribute to the risk of alcohol use disorder (AUD), by integrating large-scale GWAS data with RNA-seq data derived from post-mortem brain tissues (Molecular Psychiatry, 2023).
Other research areas
We have extensive experience in the methodology development and applications on next generations sequencing technology, and computational modeling on transcriptional regulation, microRNA regulation, and epigenetic regulation. Our team has recently invested heavily on developing novel computational methods for single-cell analytics and spatial transcriptomics technologies.
Current Research Funding
1R01DA053722, 09/30/21 – 06/30/26, NIH-NIDA, Role: MPI
Functional genetic variants in substance use disorders
The goal of this project is to design and implement a set of massively parallel reporter assays and computational methods for prioritizing causal genetic variants and identifying genes that contribute to substance use disorders.
3R01DA053722-03S1 , 07/01/23 – 06/30/24, NIH-NIDA, Role: MPI
Functional genetic variants in Alzheimer’s disease
As the administrative supplement of the parent grant, the goal of this project is to design and implement a set of massively parallel reporter assays and computational methods for prioritizing causal genetic variants and identifying genes that contribute to Alzheimer’s disease.
1R25HG012325, 08/10/22 – 05/31/27, NIH-NHGRI, Role: contact PI
Indiana Genomics Research Training Program for Data Scientists (INGEN4DS)
The Indiana Genomics Research Training Program for Data Scientists (INGEN4DS) will provide practical training and hands-on research experience in genomics data analysis to prepare and encourage a diverse pool of talented data science master’s degree students to enter the genomics workforce.
2P30CA082709, 09/01/19 – 08/31/24, NIH-NCI, Role: PI
Center for Medical Genomics, Indiana University Simon Comprehensive Cancer Center
The Genomics Core (GC) at Indiana University School of Medicine (IUSM) provides state-of-the-art genomics services to investigators at Indiana University Simon Cancer Center (IUSCC), including next generation sequencing, single-cell analytics, microarray and high-throughput genotyping.
2U54CA196519, 07/01/21 – 08/31/26, NIH-NCI, Role: PI
Developmental and Hyperactive Ras Tumor (DHART) SPORE: Omics Core
The overall goal of the Omics Core (Core B) is to provide state-of-the-art support for research design consultation, performance of genomics and kinomics experiments, and integrated data analysis to DHART investigators to elucidate changes during NF1-related tumor development and in response to therapy.
1R56NS129438-01A1, 09/01/23 – 08/31/24, NIH-NINDS, Role: MPI
Immune checkpoints in the CNS and HIV-associated neurocognitive disorder
Our research aims to study the levels, profiles, and clinical relevance of immune checkpoints (ICPs), key regulators of immune homeostasis and defense, in the brain of HIV-infected people with or without neurocognitive disorder (HAND).
1U19AG074879, 03/15/23 – 02/29/28, NIH-NIA, Role: MPI
The Omics core will support the overall U19 CLEAR-AD (Centrally-linked longitudinal peripheral biomarkers of AD in multi-ethnic populations) program by generating multiple layers of omics data from biospecimens collected as part of the well characterized cohorts of ADNI, MCSA and 5 ADRCs (Mayo Clinic, Indiana University, 1Florida, University of Michigan and Washington University).
Recent Publications
Li R, Reiter JL, Chen AB, Chen SX, Foroud T, Edenberg HJ, Lai D, Liu Y. RNA alternative splicing impacts the risk for alcohol use disorder. Mol Psychiatry. 2023 May 23. doi: 10.1038/s41380-023-02111-1. Epub ahead of print. PMID: 37217680.
Chen SX, Simpson E, Reiter JL, Liu Y. Bioinformatics detection of modulators controlling splicing factor-dependent intron retention in the human brain. Hum Mutat. 2022 Nov;43(11):1629-1641. doi: 10.1002/humu.24379. Epub 2022 May 10. PMID: 35391504; PMCID: PMC9537345.
Dong C, Reiter JL, Dong E, Wang Y, Lee KP, Lu X, Liu Y. Intron-Retention Neoantigen Load Predicts Favorable Prognosis in Pancreatic Cancer. JCO Clin Cancer Inform. 2022 Feb;6:e2100124. doi: 10.1200/CCI.21.00124. PMID: 35148169; PMCID: PMC8846286.
Rao X, Thapa KS, Chen AB, Lin H, Gao H, Reiter JL, Hargreaves KA, Ipe J, Lai D, Xuei X, Wang Y, Gu H, Kapoor M, Farris SP, Tischfield J, Foroud T, Goate AM, Skaar TC, Mayfield RD, Edenberg HJ, Liu Y. Allele-specific expression and high- throughput reporter assay reveal functional genetic variants associated with alcohol use disorders. Mol Psychiatry. 2021 Apr;26(4):1142-1151. doi: 10.1038/s41380-019-0508-z. Epub 2019 Sep 2. PMID: 31477794; PMCID: PMC7050407.
Dong C, Cesarano A, Bombaci G, Reiter JL, Yu CY, Wang Y, Jiang Z, Zaid MA, Huang K, Lu X, Walker BA, Perna F, Liu Y. Intron retention-induced neoantigen load correlates with unfavorable prognosis in multiple myeloma. Oncogene. 2021 Oct;40(42):6130-6138. doi: 10.1038/s41388-021-02005-y. Epub 2021 Sep 9. PMID: 34504297; PMCID: PMC8426332.
Research Team

Yunlong Liu, PhD, MS
Director, Center for Computational Biology & Bioinformatics
Other research team members in the Liu Lab include Jill Reiter, PhD (Visiting Assistant Research Professor), Wenting Wu, PhD (Assistant Research Professor), Hongyu Gao, PhD (Assistant Scientist), Guanglong Jiang, PhD (Bioinformatics Analyst), Rudong Li, PhD (Post-doctoral Fellow), Andy B. Chen, PhD (Post-doctoral Fellow), Muyi Liu, PhD (Post-doctoral Fellow), Xiaona Chu (Research Analyst/Technician), Kerry Sanders (Research Analyst/Technician), Edward R. Simpson, Steven Chen, Duojiao Chen, and Chuanpeng Dong.