The major focus of Thomas D. Hurley, PhD's research lab is to understand, at the molecular level, the processes involved in the recognition and binding of molecules that are directed to the active sites of enzymes. The main approaches utilized in this laboratory are X-ray crystallography, inhibitor screening, detailed enzyme kinetics and mass spectrometry.
Active Research
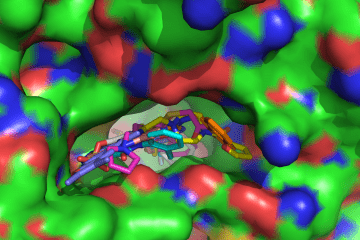 Aldehyde Dehydrogenases
Aldehyde Dehydrogenases
A major focus of this study area is to correlate the structural and functional characteristics of aldehyde dehydrogenases as they impact cellular function. The goal is to understand the catalytic function of this important enzyme family and how manipulation of its activity through the design of small molecule modulators of its activity can affect disease outcomes. In particular, investigators in this laboratory have become interested in the roles of aldehyde dehydrogenases from family 1A (ALDH1A) in chemoresistance in certain forms of cancer and as biomarkers for cancer stem cells, also known as cancer initiating cells. Toward this end, the lab team has pursued the discovery and development of small molecule inhibitors that demonstrate selectivity toward the different enzyme that is used as chemical tools to probe the manner in which these enzymes impact cancer cell survival.
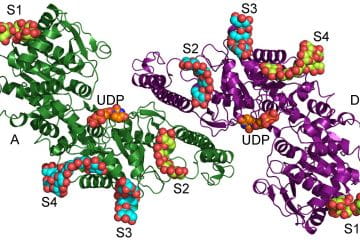 Glycogen Synthesis
Glycogen Synthesis
The second major research direction of this lab is in collaboration with Peter Roach, PhD, and Anna Depaoli-Roach, PhD, where investigators are exploring the structural and functional properties of the major enzyme that controls glycogen synthesis; glycogen synthase. In particular, the team is are interested in how manipulation of glycogen synthase can impact the outcome of diseases associated with the over-accumulation of glycogen – so called glycogen storage diseases, such as Pompe and Lafora Disease. The goal of this collaboration is to discover and develop novel inhibitors that can block further accumulation of glycogen in cellular and mouse model systems of these diseases. To facilitate the discovery and development of the novel inhibitors our laboratory is using the structure and enzyme characteristics of glycogen synthase to inform both the medicinal chemistry and biological evaluation in cell and animal models from our collaborators.
Research Funding
R01DK27221-37 (Roach and Hurley, MPI)
Small Molecule Inhibition of Glycogen Storage as Therapy of Glycogenoses
1R01CA214567-01 (PI: Larson; Hurley- CoI)
Isozyme-selective ALDH Inhibitors for Sensitizing Ovarian Cancer Stem-like Cells to Chemotherapy
P01NS097197-01 Project #3: (Leader: P Roach/Hurley – Co-I)
Suppressing glycogen storage with small molecule inhibitors as a therapeutic approach to LD
Define the clinical biochemistry of LD mutations to provide a personalized diagnosis and establish therapeutic options to treat LD, ultimately resulting in a cure.
Recent Publications
A full list of publications by Thomas D. Hurley, PhD, are available on PubMed.
